
COVID-19, Classifications, and Definitions
-
by bytethebuzz
- 2300
Viruses like COVID-19 (SARS-CoV-2) continuously evolve as changes in the genetic code (caused by genetic mutations or viral recombination) occur during the replication of the genome. A lineage is a genetically closely related group of virus variants derived from a common ancestor. A variant has one or more mutations that differentiate it from other variants of the SARS-CoV-2 viruses. A recombinant is a variant created by the combination of genetic material from two different variants. As expected, multiple variants of SARS-CoV-2 have been documented in the United States and Globally throughout this pandemic. To inform local outbreak investigations and understand national trends, scientists compare genetic differences between viruses to identify variants (including recombinants) and how they are related to each other.
Key Definitions:
- Mutation: A mutation refers to a single change in a virus’s genome (genetic code). Mutations happen frequently, but only sometimes change the characteristics of the virus.
- Recombinant: A process in which the genomes of two SARS-CoV-2 variants (that have infected a person at the same time) combine during the viral replication process to form a new variant that is different from both parent lineages.
- Lineage: A lineage is a group of closely related viruses with a common ancestor. SARS-CoV-2 has many lineages; all cause COVID-19.
- Variant: A variant is a viral genome (genetic code) that may contain one or more mutations. In some cases, a group of variants with similar genetic changes, such as a lineage or group of lineages, may be designated by public health organizations as a Variant Being Monitored (VBM), Variant of Concern(VOC) or a Variant of Interest (VOI) due to shared attributes and characteristics that may require public health action.
Key Points
Genetic lineages of SARS-CoV-2 have been emerging and circulating around the world since the beginning of the COVID-19 pandemic.
SARS-CoV-2 genetic lineages in the United States are routinely monitored through epidemiological investigations, virus genetic sequence-based surveillance, and laboratory studies.
On November 30, 2021, the U.S. government SARS-CoV-2 Interagency Group (SIG) classified Omicron as a Variant of Concern(VOC). This classification was based on the following:
Detection of cases attributed to Omicron in multiple countries, including among those without travel history.
Transmission and replacement of the Delta variant in South Africa.
The number and locations of substitutions in the spike protein.
Available data for other variants with fewer substitutions in the spike protein that indicate a reduction in neutralization by sera from vaccinated or convalescent individuals.
Available data for other variants with fewer substitutions in the spike protein that indicate reduced susceptibility to certain monoclonal antibody treatments.
On April 14, 2022 the U.S government SARS-CoV-2 Interagency Group (SIG) downgraded Delta from a Variant of Concern to a Variant Being Monitored This new classification was based on the following:
Significant and sustained reduction in its national and regional proportions over time.
Evidence suggests that Delta does not currently pose a significant risk to public health in the United States.
The SIG Variant classification scheme defines four classes of SARS-CoV-2 variants:
Variant Being Monitored (VBM)
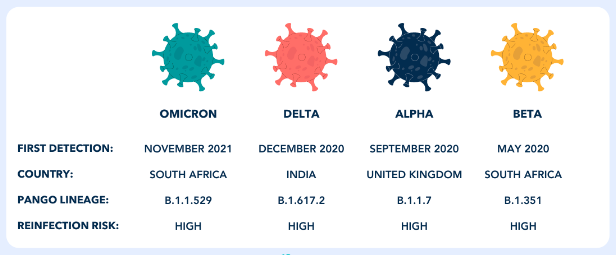
- Alpha (B.1.1.7 and Q lineages)
- Beta (B.1.351 and descendent lineages)
- Gamma (P.1 and descendent lineages)
- Delta (B.1.617.2 and AY lineages)
- Epsilon (B.1.427 and B.1.429)
- Eta (B.1.525)
- Iota (B.1.526)
- Kappa (B.1.617.1)
- 1.617.3
- Mu (B.1.621, B.1.621.1)
- Zeta (P.2)
Variant of Interest (VOI)
Variant of Concern (VOC)
- Omicron (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4 and BA.5 lineages)
Variant of High Consequence (VOHC)
- To date, no variants of high consequence have been identified in the United States.
- Vaccines approved and authorized for use in the United States are effective against the predominant variant circulating in the United States and effective therapeutics are available. CDC continues to monitor all variants circulating within the United States.
How Variants Are Classified
The U.S. Department of Health and Human Services (HHS) established a SARS-CoV-2 Interagency Group (SIG) to enhance coordination among CDC, National Institutes of Health (NIH), Food and Drug Administration (FDA), Biomedical Advanced Research and Development Authority (BARDA), and Department of Defense (DoD). This interagency group is focused on the rapid characterization of emerging variants and actively monitors their potential impact on critical SARS-CoV-2 countermeasures, including vaccines, therapeutics, and diagnostics.
The SIG meets regularly to evaluate the risk posed by SARS-CoV-2 variants circulating in the United States and to make recommendations about the classification of variants. This evaluation is undertaken by a group of subject matter experts who assess available data, including variant proportions at the national and regional levels and the potential or known impact of the constellation of mutations on the effectiveness of medical countermeasures, severity of disease, and ability to spread from person to person. Given the continuous evolution of SARS-CoV-2 and our understanding of the impact of variants on public health, variants may be reclassified based on their attributes and prevalence in the United States.
- Variants Being Monitored (VBM)– View current VBM in the United States that continue to be monitored and characterized by federal agencies
- Variant of Interest (VOI)– Currently, no SARS-CoV-2 variants are designated as VOI
- Variant of Concern (VOC)– View current VOC in the United States that are being closely monitored and characterized by federal agencies
- Variant of High Consequence (VOHC)– Currently, no SARS-CoV-2 variants are designated as VOHC
Notes: Each variant classification includes the possible attributes of lower classes (for example, VOC includes the possible attributes of VOI); variant status might escalate or deescalate based on emerging scientific evidence. This page will be updated as needed to show the variants that belong to each class. The World Health Organization (WHO) also classifies variant viruses as variants of concern and variants of interest; U.S. classifications may differ from those of WHO because the impact of variants may differ by location. To assist with public discussions of variants, WHO proposed using labels consisting of the Greek alphabet (for example, alpha, beta, gamma) as a practical way to discuss variants for non-scientific audiences. The labels assigned to each variant are provided in the tables below.
Variants Being Monitored (VBM)
CDC monitors all variants circulating in the United States. Variants designated as VBM include those where data indicates there is a potential or clear impact on approved or authorized medical countermeasures or that have been associated with more severe disease or increased transmission but are no longer detected, or are circulating at very low levels, in the United States. These variants do not pose a significant and imminent risk to public health in the United States.
A Variant of Interest or a Variant of Concern may be downgraded to this list after a significant and sustained reduction in its national and regional proportions over time, or other evidence indicates that a variant does not pose significant risk to public health in the United States.
These variants continue to be closely monitored to identify changes in their proportions and new data are continually being analyzed. If the data indicate that a VBM warrants more concern, the classification will be changed based on the SIG assessment of the attributes of the variant and the risk to public health in the United States.
| WHO Label | Pango Lineage | Date of Designation | Date of Designation | Date of Designation |
| Alpha | B.1.1.7 and Q lineages | VOC: December 29, 2020 | VBM: September 21, 2021 | |
| Beta | B.1.351 and descendent lineages | VOC: December 29, 2020 | VBM: September 21, 2021 | |
| Gamma | P.1and descendent lineages | VOC: December 29, 2020 | VBM: September 21, 2021 | |
| Delta | B.1.617.2 and AY lineages | VOC: June 15, 2021 | VBM: April 14, 2022 | |
| Epsilon | B.1.427 B.1.429 | VOC: March 19, 2021 | VOI: February 26, 2021 VOI: June 29, 2021 | VBM: September 21, 2021 |
| Eta | B.1.525 | VOI: February 26, 2021 | VBM: September 21, 2021 | |
| Iota | B.1.526 | VOI: February 26, 2021 | VBM: September 21, 2021 | |
| Kappa | B.1.617.1 | VOI: May 7, 2021 | VBM: September 21, 2021 | |
| N/A | B.1.617.3 | VOI: May 7, 2021 | VBM: September 21, 2021 | |
| Zeta | P.2 | VOI: February 26, 2021 | VBM: September 21, 2021 | |
| Mu | B.1.621, B.1.621.1 | VBM: September 21, 2021 |
Variant of Interest (VOI)
A variant with specific genetic markers that have been associated with changes to receptor binding, reduced neutralization by antibodies generated against previous infection or vaccination, reduced efficacy of treatments, potential diagnostic impact, or predicted increase in transmissibility or disease severity.
Possible attributes of a Variant of Interest:
- Specific genetic markers that are predicted to affect transmission, diagnostics, therapeutics, or immune escape.
- Evidence that it is the cause of an increased proportion of cases or unique outbreak clusters.
- Limited prevalence or expansion in the US or in other countries.
A Variant of Interest might require one or more appropriate public health actions, including enhanced sequence surveillance, enhanced laboratory characterization, or epidemiological investigations to assess how easily the virus spreads to others, the severity of disease, the efficacy of therapeutics and whether currently approved or authorized vaccines offer protection.
Variant of Concern (VOC)
A variant for which there is evidence of an increase in transmissibility, more severe disease (for example, increased hospitalizations or deaths), significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures.
Possible attributes of a variant of concern:
In addition to the possible attributes of a variant of interest
- Evidence of impact on diagnostics, treatments, or vaccines
- Widespread interference with diagnostic test targets
- Evidence of substantially decreased susceptibility to one or more class of therapies
- Evidence of significantly decreased neutralization by antibodies generated during previous infection or vaccination
- Evidence of reduced vaccine-induced protection from severe disease
- Evidence of increased transmissibility
- Evidence of increased disease severity
Variants of concern might require one or more appropriate public health actions, such as notification to WHO under the International Health Regulations, reporting to CDC, local or regional efforts to control spread, increased testing, or research to determine the effectiveness of vaccines and treatments against the variant. Based on the characteristics of the variant, additional considerations may include the development of new diagnostics or the modification of vaccines or treatments.
Current variants of concern in the United States that are being closely monitored and characterized are listed below. This table will be updated when a new variant of concern is identified.
a – Phylogenetic Assignment of Named Global Outbreak (PANGO) Lineages is software tool developed by members of the Rambaut Lab. The associated web application was developed by the Centre for Genomic Pathogen Surveillance in South Cambridgeshire and is intended to implement the dynamic nomenclature of SARS-CoV-2 lineages, known as the PANGO nomenclature.
b – Nextstrain, a collaboration between researchers in Seattle, USA and Basel, Switzerland, provides open-source tools for visualizing the genetics of outbreaks. The goal is to support public health surveillance by facilitating understanding of the spread and evolution of pathogens.
Characteristics of Selected SARS-CoV-2 Variants
WHO Label: Omicron
Pango Lineage: B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4 and BA.5 lineages (Pango Lineage)a
Spike Protein Substitutions: A67V, del69-70, T95I, del142-144, Y145D, del211, L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F
Nextstrain clade (Nextstrain)b: 21K
First Identified: South Africa
Attributes:
- Potential increased transmissibility
- Potential reduction in neutralization by some EUA monoclonal antibody treatments
- Potential reduction in neutralization by post-vaccination sera
Variant of High Consequence (VOHC)
A VOHC has clear evidence that prevention measures or medical countermeasures (MCMs) have significantly reduced effectiveness relative to previously circulating variants.
Possible attributes of a variant of high consequence:
In addition to the possible attributes of a variant of concern
- Impact on MCMs
- Demonstrated failure of diagnostic test targets
- Evidence to suggest a significant reduction in vaccine effectiveness, a disproportionately high number of infections in vaccinated persons, or very low vaccine-induced protection against severe disease
- Significantly reduced susceptibility to multiple EUA or approved therapeutics
- More severe clinical disease and increased hospitalizations
A variant of high consequence would require notification to WHO under the International Health Regulations, reporting to CDC, an announcement of strategies to prevent or contain the transmission, and recommendations to update treatments and vaccines.
Viruses like COVID-19 (SARS-CoV-2) continuously evolve as changes in the genetic code (caused by genetic mutations or viral recombination) occur during the replication of the genome.
Viruses like COVID-19 (SARS-CoV-2) continuously evolve as changes in the genetic code (caused by genetic mutations or viral recombination) occur during the replication of the genome.